Dark side of IV fluids?
Septic patients are typically hypotensive and low blood pressure is often associated with an increased blood lactate concentration, a low urinary output, and other signs and symptoms of physiological distress. Clinicians then make an inference that there must be inadequate vital organ blood flow (typically described as poor perfusion) to explain this clinical picture. An additional inference is then made that that the patient’s cardiac output must be low because the vasodilatation, which is responsible for the patient’s low blood pressure, must have decreased venous return and thereby preload. Then rapid bolus fluid resuscitation becomes physiologically imperative and the need for its administration becomes so obvious. Such fluid bolus becomes the best means to increase cardiac output and improve organ perfusion and blood pressure. Then, we also assume that increasing cardiac output is both necessary and life-saving but using vasopressor drugs or restrictive fluid therapy with vasopressors to achieve them would lead to inferior patient outcomes.
Many fundamental questions arise out of those assumptions. For example, does fluid bolus resuscitation in patients with severe sepsis actually increase vital organ blood flow or even cardiac output? If it does, what is the magnitude of its effect on both the blood flow and cardiac output for a given dose? How long does this effect last? What is the physiological price (positive fluid balance, worse gas exchange, organ edema, acid–base changes) paid to achieve this effect? What happens to the organ perfusion after a fluid bolus? What happens if we don’t give a bolus?
Why do people give fluids?
In United States, the most common reason for fluid bolus is hypotension and oliguria. Fluid bolus aims to increase the cardiac output (CO), and this occurs only in ‘‘preload-dependent’’ patients. It’s interesting to note that injudicious administration of the very fluids, which could lead to fluid overload, thereby causing refractory hypotension and oliguria, due to its effects on cardiovascular and renal system.
When either hypotension or oliguria is present, most people only think of hypovolemia as the sole cause and not consider any other possibilities. A vague order is then given, “let's give some fluid and see what happens.” But what does “see what happens” really mean? Quite often, we think of an improvement in blood pressure to be a positive response. However, does an improvement in blood pressure actually mean any improvement in stroke volume or cardiac output? Does any improvement in urine output means any increase in stroke volume? Or vice versa? This approach often results in the infusion of large amounts of fluid (fluid loading) without any real understanding of whether this fluid is actually needed.
This has led to the problem of fluid overload in epidemic proportions.
Fluid Overload and mortality:
When used appropriately i.v. fluids can obviously improve outcomes. However, in view of the physiological complexity of fluid resuscitation, many physicians prescribing fluid therapy appear to lack cognition for its potential to cause harm.
Study after the study has shown strong evidence that fluid overload is associated with worse outcomes. However, we still see people carelessly and recklessly use IV fluids without any rationale. Even worse, people seem to be not bothered by fluid overload, unless patient is hypoxic. An emerging evidence to tackle this problem use an approach of 4D’s or ROSE approach.
During early phases of sepsis, patients are given multiple boluses of IV fluids as part of early goal-directed therapy. Fluid over load is usually the result of injudicious and overenthusiastic resuscitation during late phases of sepsis.
What can we do to prevent fluid overload:
All too often, the ‘recipe’ fluid therapy that is ‘one size (dose) fits all’ is chosen for reasons of convenience or possibly because clinicians do not actually think about why they are giving fluids in the first place. To prevent this epidemic of fluid overload, institutions need to adopt fluid stewardship, similar to antibiotic stewardship. One very popular approach is 4D's approach or ROSE approach.
ROSE approach: An emerging approach to tackle the menace of fluid overload. It involves for phases of critical illness involving IV fluids
-
Resuscitation phase (R): It is a rescue phase where the goal is to urgently treat and prevent hypo perfusion/shock induced by hypovolemia. During early phases of severe sepsis/septic shock, current guidelines recommend at least 30 mL/Kg fluid resuscitation. Typically, fluids are given as boluses and this phase should last no longer than a few minutes to an hour. Patients are in 2-3 L positive balance by the end of this phase.
-
Optimization phase (O): The main goal of this phase is organ rescue and optimize tissue and organ perfusion. This occurs within hours after the early resuscitation phase. During this phase, the patient is no longer in immediate life-threatening danger but is in a stage of compensated shock and any additional fluid therapy is given more cautiously, depending on the level of fluid tolerance. Typically, patients would get further fluid boluses, depending on dynamic variables and fluid responsiveness. This phase will last a few hours to a day. People who are in more than 3 L positive balance within first 24 hours have poor outcomes.
-
Stabilization phase (S): The main goal of this phase is organ support and this usually evolves over a few days. Patients are hemodynamically stable during phase. The goal is to keep patient’s fluid balance even during this phase. Patients should be on maintenance fluids only if they have ongoing fluid losses or if their oral intake is poor. Avoid overenthusiastic fluid therapy during this phase and minimize the risk of fluid overload.
-
Evacuation phase (E): Also called as deresuscitation phase or organ recovery phase. This phase requires a late goal-directed fluid removal to achieve negative fluid balance. A goal of net 0 to negative fluid balance by day 3 should be targeted. Typically, RRT or diuretics (sometimes in combination with albumin) can be used to mobilize the excess volume. In some patients, this phase could last days to weeks.
Typically, most patients requiring fluid resuscitation will enter this conceptual framework in the Rescue phase. However, some (especially already admitted floor patients) may enter at the Optimization phase, where fluid challenges rather than fluid boluses are the initial management. Some patients may even enter in stabilization phase and would not benefit from additional fluid administration. All patients will then proceed to next phase as their clinical condition improves, and the prioritization for fluid management now switches to prevention of its adverse effects.
Fluid balance and use of diuretics / RRT:
Fluid overload is associated with worse outcomes, including the possibility of decreased renal recovery in critical care patients. It is therefore important to understand that fluid therapy in the critical care unit is a dynamic process. Efforts should be made to find a balance between giving sufficient fluid therapy to maintain hemodynamic stability and organ perfusion while avoiding overzealous volume administration. This may be achieved by initial goal-directed resuscitation during acute presentation and thereafter aiming for a neutral or slightly negative fluid balance. If fluid therapy is indicated, as in true hypovolemia, crystalloids should be favored. Synthetic colloids should be avoided, considering the extensive data regarding their safety profile and lack of clear clinical benefits. Diuretics should be used as adjunctive therapy in AKI to treat fluid overload and possibly to prevent it; however, they should not be continued if there is no an adequate response. Patients with significant fluid accumulation and who are unresponsive to diuretics should be considered for early initiation of RRT to correct fluid overload.
Adverse effects of fluid overload:
Large volume fluid resuscitation results in severe tissue edema and clinical signs of volume overload. Tissue edema impairs oxygen and metabolite diffusion, distorts tissue architecture and impedes capillary blood flow and lymphatic drainage. These effects are pronounced in encapsulated organs, such as the liver and kidneys, which lack the capacity to accommodate additional volume without an increase in interstitial pressure, resulting in compromised organ blood flow.
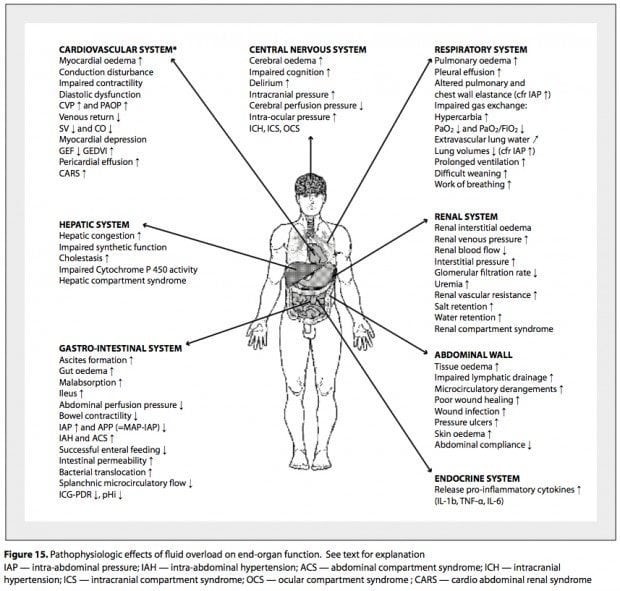
Effect of fluid overload on multiple organ systems include:
-
CNS: Cerebral edema, impaired cognition, delirium, elevated ICP
-
Cardiac: Increased myocardial edema, myocardial depression, impaired contractility, decreased stroke volume with decreased cardiac output, diastolic dysfunction, increased CVP and wedge pressure, decreased venous return, and pericardial effusion.
-
Pulmonary: Pulmonary edema, pleural effusions, impaired gas exchange, decreased lung compliance, hypoxia, hypercapnia, increased extravascular lung water, increased work of breathing, prolonged ventilation, difficulty weaning.
-
GI: Ascites, hepatic congestion, increased portal vein pressure, increased ammonia, decreased lactate clearance, gut edema with mild absorption, increased intestinal permeability, increased bacterial translocation, intra-abdominal hypertension with decreased splanchnic circulatory flow, ileus and decreased bowel contractility.
-
Renal: Increased renal vein pressure, Increased renal interstitial edema, renal compartment syndrome, increased ADH secretion, increased renal interstitial pressure with decreased renal blood flow, decreased GFR, oliguria with resultant acute kidney injury.
-
Musculoskeletal: Increased tissue edema, impaired lymphatic drainage, poor wound healing, pressure ulcers, and decreased thoraco-abdominal compliance.
-
Hematology: Dilutional coagulopathy, anemia (studies have found that Hb is diluted by 1 gm for each liter of positive fluid balance).
Could fluids harm a septic patient?
There is great evidence showing fluid overload associated with increased mortality. Many factors affect the mortality and morbidity of the patient from fluid overload. Even though, fluids for typically given to improve cardiac output, it could be counterproductive and can actually worsen the cardiac output. Fluids could harm the patient in one of the following ways
-
Fluid bolus could result in greater rise in CVP than the mean circulatory filling pressure, as venous system is typically very compliant and has a lot of capacity to accommodate additional volume. Then, there will be a decreased gradient between MCFP and CVP resulting in decreased venous return. Decreased venous return leads to decreased cardiac output. So, the very fluid which is given to increase the cardiac output could actually end up worsening the cardiac output and microcirculatory perfusion.
-
When fluid bolus results in greater increase in CVP, thereby stretching the right atrium, it also releases natriuretic peptide. Natriuretic peptides cleave membrane-bound proteoglycans and glycoproteins off the endothelial glycocalyx. Damage to the glycocalyx profoundly increases endothelial permeability, leading to more capillary leak with intravascular volume depletion. This leads to a vicious cycle of fluid boluses, leading to more capillary leak, with resultant further decrease in intravascular volume leading to a drop in cardiac output.
-
Sepsis is a vasoplegic state with both arterial and venodilation. Arterial dilatation results in systemic hypotension. However, more importantly, profound venodilation occurs in the splanchnic and cutaneous vascular beds increasing the unstressed blood volume, decreasing venous return and cardiac output. It is best treated with vasopressors which could cause venoconstriction and increase stressed volume, which will lead to increased venous return and cardiac output. If we use fluids instead of vasopressors, it may not lead to increase in stressed volume and possibly has no effect on venous return/cardiac output.
-
Sepsis induced cardiomyopathy: Sepsis could induce a temporary myocardial depression with a resultant low EF. Fluids could be very dangerous in this state of acute systolic heart failure.
-
Fluid responsiveness: In non-responders, volume loading serves the patient no useful benefit and is likely harmful. The adverse effects of fluid loading when a patient is on the flat portion of the Frank-Starling curve, is related to the increase in atrial pressures, thereby leading to increased release of natriuretic peptides, causes a shift of fluid into the interstitial space, with an increase in pulmonary and tissue edema. Tissue edema impairs oxygen delivery and impedes microcirculatory flow. The kidney is particularly affected by increased venous pressure, which leads to increased renal sub capsular pressure and reduced renal blood flow and GFR.
-
The goal of fluid resuscitation is to increase the stressed blood volume and MCFP more than the CVP, and thereby increase the pressure gradient for venous return. However, hemodynamic effects of a fluid bolus (in the fluid responders) are short-lived, with the net effect being the shift of fluid into the interstitial compartment with tissue edema. In a study, only 15% of a crystalloid bolus remained in the intravascular space at 3 h, with 50% of the infused volume being in the extravascular space. Ann Surg. 2012 Jul;256(1):18-24
-
Blood pressure responsiveness is often mistaken as fluid responsiveness. In a study, 67% of patients were fluid responders, however the MAP increased in only 44% of these patients (pressure responder). Intensive Care Med. 2015 Jul;41(7):1247-55 , Med Intensiva. 2017 Dec;41(9):546-549
-
Fluids as vasodilators: Some studies have demonstrated a decrease in SVR after fluid resuscitation in patients with sepsis. This suggests that fluid boluses should be considered vasodilator therapy, in patients with sepsis and that aggressive fluid resuscitation may potentiate the hyperdynamic state. Intensive Care Med. 2012 Mar;38(3):422-8 ,
Common misconceptions about fluids in sepsis:
-
Blood pressure as the target of fluid resuscitation: A reliable detection of preload dependency is often challenging, and a fluid challenge is often performed based on ‘‘let’s give fluids and see what happens’’ approach. Ideally, this strategy requires measuring CO to ensure that CO has actually increased after a fluid challenge. As hypotension is a common trigger for volume expansion, clinicians are often tempted to use blood pressure (BP) changes as a very simple surrogate for CO changes. An increase in BP during fluid challenge is deemed to reflect an increase in CO, whereas, if the BP remains low, the patient has probably not responded. Indeed, if the arterial tree properties (arterial impedance) remain unchanged during the fluid challenge, one could expect that changes in BP and CO may be closely related. But, there was no correlation between change in blood pressure and cardiac output. However, there is a weak correlation between increase in cardiac output and increase in pulse pressure (>23 % for invasive and 35 % for non-invasive blood pressure monitoring). Pulse pressure increase less than 5% reliably ruled out fluid responsiveness. Intensive Care Med. 2013 Nov;39(11):1953-62
-
Oliguria in sepsis: Often it is presumed that low urine output in a septic patient is from hypovolemia. AKI in sepsis is often not from hypo perfusion. In fact, kidneys are hyperemic in sepsis and often has more than enough cardiac output. There is an efferent arteriole vasodilation resulting in decreased filtration pressure and thereby GFR. After the initial resuscitation, fluid administration may not increase urinary output and contributes to positive fluid balance and potentially worsening of AKI in patients with septic shock.
-
Fluids are safe and vasopressors are dangerous: Fluids are thought to be benign drugs and vasopressors are considered very dangerous. People also think that if we start vasopressors too early without fluid resuscitation, it will cause hypo perfusion and organ damage. However, the evidence is exactly the opposite. Fluids have been shown to worsen AKI and increase mortality. There is not a single study which showed worse mortality/ morbidity from use of vasopressors. In fact, evidence showed that the mortality increase by 3% for every hour of delay in starting vasopressors in septic shock. Recent trials which used less fluids have shown to improve mortality. FEAST trial, CLASSIC trial, Zambian study. Ongoing studies include REFRESH study, RIFTS study, SHOCK Trial, CLOVERS trial.
-
CVP as a marker of hypo perfusion: The likelihood that CVP can accurately predict fluid responsiveness is only 56% (slightly better than flipping a coin). The correlation between change in CVP and change in cardiac output was even worse (correlation coefficient of 11%). In patients with an intact sympathetic response to hypovolemia, the CVP may actually fall in response to fluid, as compensatory venoconstriction is reduced. An excellent article on common misconceptions about CVP can be found here.
-
Lactic acidosis is a marker of hypo perfusion: We used to assume that lactate is a marker of tissue hypoxia. Accordingly, elevated lactate has been thought to indicate the presence of an 'oxygen debt' or 'hypoperfusion', which leads to increased lactate generation via anaerobic glycolysis. Current evidence now supports that lactate production is not due only to tissue hypoxia or anaerobic glycolysis but also by increased aerobic glycolysis secondary to activation of the stress response (adrenergic stimulation). More importantly, new evidence suggests that lactate may actually serve to facilitate bioenergetic efficiency through an increase in lactate oxidation. Crit Care. 2014 Sep 9;18(5):503 , OA Critical Care 2013 Mar 01;1(1):3
My approach:
I approach fluids in sepsis in 3 steps:
-
Early adequate goal directed fluid management: I usually start with 20cc/kg fluid bolus in ED patients whose volume status is unknown. If they are hypovolemic and fluid responsive, I give more fluids as boluses, each time limited to a 500ml. Obviously, clinical context must be taken into account. More fluid is needed in septic shock from peritonitis than from pneumonia.
-
Late Conservative Fluid Management: Once I finish the upfront fluid resuscitation until they are euvolemic, I then only give maintenance fluids if they have ongoing fluid losses or if they are NPO. My aim is to maintain net even balance during this phase. Recent studies have shown that late conservative fluid management, defined as two consecutive days of negative fluid balance within the first week of ICU stay, is a strong and independent predictor of survival.
-
Late Goal Directed Fluid Removal (LGFR). Once, their hemodynamics are stable, I start more aggressive and active fluid removal by means of diuretics or RRT with net ultrafiltration, if needed. This is referred to as ‘de-resuscitation’. I take De-resuscitation as seriously as Resuscitation phase. My goal is a net zero by day 3, which is consistent with evidence.
Conclusion:
-
Ideally, fluid resuscitation should be guided by the determination of fluid responsiveness rather than a reflex action without any thought process. Only monkeys do that.
-
Initial resuscitation of patients with septic shock should logically include at most 500 ml boluses of crystalloid (Ringer’s lactate), to a maximum of about 20 ml/kg.
-
Norepinephrine should be initiated in those patients who remain hypotensive (MAP < 65 mm Hg) despite this initial, limited fluid strategy. Venous capacitance vessels are much more sensitive to sympathetic stimulation than arterial resistance vessels. Hence, low dose α-1 agonists cause greater venoconstriction than arterio-constriction. In septic patients, α-1 agonists mobilize blood from the unstressed reservoirs in the splanchnic circulation and skin, thereby increasing venous return and cardiac output.
-
Dopamine is a very weak vasopressor and should never be used as a vasopressor in septic shock. J Intensive Care Med. 2012 May-Jun;27(3):172-8 , Crit Care Med. 2012 Mar;40(3):725-30 , Crit Care Med. 2015 Oct;43(10):2141-6 .
-
In patients admitted to hospital and whose volume status is already known, sudden onset hypotension or oliguria should not lead to knee jerk reaction of a fluid bolus.
-
Ab“normal” solution a.k.a normal saline should never be used in sepsis. There is nothing normal about normal saline.
-
I.V. fluid therapy can be lifesaving but like all medical interventions carries with it a degree of risk. IV fluid therapy should be considered as a drug therapy with risks and benefits. Just like any medication, we should not be administering IV fluids unless we understand them properly.
A must read article for everyone who is using IV fluids in sepsis is here.
PEARLS:
Excellent article Dr Nandyala, and much needed education!
Most overload happens in two phases.
1. Overzealous boluses in beginning
2. By " maintaining"
Lastly, there is a hurry to stop vasopressors or reluctance to start.
Opposite end of this scenario is in patients with chf or esrd, they get no fluids.
I guess the amount of fluid to be given becomes a "judgement" call, and moet people don't know how to judge.
Very well said. Fluid creep / maintainence fluids has become like an epidemic now.
Sir please explain about bicar icu trail
Hello,
Waiting for new posts. It's been a while.
Dr. Nandyala,
Good evening.
I have some questions about pancreatitis with leukocytosis but no other criteria for SIRS, superimposed by respiratory depression due to opiods but mostly due to benzodiazepine intoxication (as evidenced by airway obstruction and increased work of breathing), with pulmonary edema resulting from aggressive fluid resuscitation (elevations in blood pressure with systolic in the 170s, RR in the 40s, tachycardia with HR > 125, and restlessness/agitation.
No signs of infection, no fevers.
Hypersensitivity reaction to Omnipaque from initial CT also seemed to develop over the first 2 days with urticaric rash on torso and right forearm.
Dropping hematocrit and hemoglobin (due to hemodilution) increase in BUN, normal lactate within 15 hours of presenting to ED. On physical exam, peripheral edema (swollen feet, legs, arms, abdominal distension). On blood gases, PaO2 was 66 at 15 hours after arrival to ED.
Why did I deteriorate within 15 hours of presenting to ED requiring mechanical ventilation for 10 days in the ICU? What evidence do I have that I experienced respiratory failure with hypoxemia primarily due to fluid overload? By day 3 following admission, a 20lb increase in weight was noted. How did the the initial hypoventilation due to the 14mg of Ativan for “agitation” administered during the first 15 hours before I was intubated contribute to the respiratory failure?
On day 4, ascites and anasarca on CT as well as pleural effusions. Creatinine doubled on day 3. Furosemide started on day 5 with clinical improvement. On day 5, cultures came back for Strep pneumo and antibiotics were started. Triglycerides were in the 1000s but not checked until 2 days after I presented. The “hyponatremia” noted by the hospitalist on admission was a pseudohyponatremia due to the hypertriglyceridemic pancreatitis which was never diagnosed or treated. There is also evidence of benzodiazepine-induced delirium and cerebral edema. Normal saline was the chosen fluid. LR was added on day 3.
I can provide additional information including results of imaging studies.
Please let me know your thoughts. Why did I deteriorate within my first 15 hours due to fluid overload and not ARDS (from pancreatits) or aspiration pneumonia? Thank you.
I suspect, the respiratory distress I experienced was soley due to compressive atelectasis and pulmonary edema causing V/Q mismatch.
Thank you.