Asthma
-
A chronic inflammatory disease of airways, characterized by increased responsiveness of the tracheobronchial tree to various stimuli
-
Bronchospasm is a secondary phenomenon caused by the underlying inflammatory process.
-
Manifested physiologically by widespread narrowing of the air passages, and clinically by paroxysms of dyspnea, cough, chest tightness and wheezing
Etiology
-
Airway hyperresponsiveness to both specific and nonspecific stimuli is the hallmark of asthma.
-
Etiology is unknown, but genetic susceptibility and airway inflammation are believed to play fundamental roles.
-
Cells thought to be important in the inflammatory response include mast cells, eosinophils, lymphocytes, and airway epithelial cells.
-
The central features of airway histopathology in asthma include infiltration of inflammatory cells, edema, subepithelial fibrosis, mucous gland and goblet cell hyperplasia, and increased airway smooth muscle mass.
-
-
Allergic (extrinsic) asthma / Atopic asthma
-
Associated with a personal and/or family history of allergic diseases, such as rhinitis, urticaria, or eczema
-
Immunoglobulin E (IgE) mediated
-
Precipitants include dust mites, pets and Seasonal pollens
-
-
Idiosyncratic (intrinsic) asthma
-
No defined immunologic mechanism
-
Precipitants include URI's, Exercise, GERD, Exposure to cold air, Tobacco smoke, Air pollutants, Drugs and Diets low in antioxidants
-
Symptoms & Signs
-
Classic symptom triad of Wheezing ( intially during expiration but later during inspiration as well), dyspnea and dry cough
-
Patients experience a sense of constriction in the chest, often with a nonproductive cough.
-
In status asthmaticus, mucus plugging and impending suffocation are signaled by lessening or disappearance of wheezing
-
Other physical findings include tachypnea, tachycardia, use of accessory muscles and pulsus paradoxus ( fall in SBP by more than 10 during inspiration. Can also be seen in cardiac tamponade and pericarditis).
Differential Diagnosis
-
Recurrent pulmonary emboli
-
Upper airway obstruction by tumor or laryngeal edema: Typically has stridor and harsh respiratory sounds can be localized to the trachea. Tracheal sounds can be transmitted to lungs and may mimic wheeze.
-
Glottic dysfunction : Narrowing glottis during inspiration and expiration produces episodic attacks of severe airway obstruction
-
Endobronchial disease (e.g., foreign-body aspiration, neoplasm, or bronchial stenosis): localised wheeze in one segment at the site of obstruction, paroxysms of cough
-
COPD
-
Systemic vasculitis with pulmonary involvement
-
Vocal cord dysfunction
-
Bronchiolitis
-
Hypersensitivity pneumonitis
-
Allergic bronchopulmonary aspergillosis ( ABPA) : IgE levels of >1000
Diagnostic Approach
-
Diagnosis of asthma is made by history and physical examination, with confirmation by pulmonary function tests.
-
Pulmonary function tests show initial airflow obstruction and reversibility with bronchodilator inhalation. Spirometry measures FVC, FEV1 and FEV1/FVC ratio.
-
Bronchoprovocation test may be required if wheezing or airflow obstruction is not initially demonstrated.
-
Peak flow measurements are useful for followup on the progression of symptoms. However, peak flow is dependant on patient effort and ehnce, not completely reliable. Record the highest of three measurements. Normal peak flow in males is between 500-650 and in females is 400-450.
Laboratory Tests
-
Complete blood count may show eosinophilia.
-
Serum IgE level : Elevated in allergic asthma, normal in idiosyncratic asthma and markedly elevated in allergic bronchopulmonary aspergillosis.
-
Sputum examination may show eosinophilia ,Curschmann’s spirals (casts of small airways) and Charcot–Leyden crystals. Presence of large numbers of neutrophils suggests bronchial infection.
-
Arterial blood gas
-
ECG may show reversible changes of right axis deviation, P pulmonale, right bundle branch block, or right ventricular hypertrophy with repolarization abnormalities
Imaging
-
Chest radiography may show hyperinflation or patchy infiltrates due to atelectasis behind plugged airways
-
Not always necessary but important when complicating infection or pneumothorax is a consideration
Pulmonary function tests
- Support the clinical diagnosis by demonstrating reversible airway obstruction.
-
Reversibility is defined as a ≥ 12% increase in FEV1 or 200ml increase in FEV1 after 2 puffs of a β-adrenergic agonist.
-
Forced vital capacity (FVC), FEV1, FEV1/FVC ratio and Peak expiratory flow rate are all decreased.
-
Residual volume and total lung capacity are increased.
-
Diffusing capacity of the lung for carbon dioxide is usually normal or slightly increased.
-
An FEV1 of < 25% predicted or < 0.75 L after administration of a bronchodilator indicates severe disease. Peak flow less than 40% or less than 200ml indicates impending respiratory failure.
-
Bronchoprovocation test may be required when spirometry results are normal and no wheezing is present
Classification
-
Mild, intermittent
-
Symptoms occur 2 or fewer times per week. Nocturnal symptoms are rare, less than twice a month.
-
FEV1 is greater than 80% predicted during episodes.
-
Predicted FEV1/FVC is normal
-
-
Mild, persistent
-
Symptoms occur more than 2 times a week but less than once a day. Nocturnal symptoms occur more than twice a month.
-
FEV1 is greater than 80% predicted during episodes.
-
Predicted FEV1/FVC is normal
-
-
Moderate, persistent
-
Symptoms occur daily. Nocturnal symptoms occur more than twice a month.
-
FEV1 is between 60% and 80% during episodes.
-
Predicted FEV1/FVC reduced by 5%
-
-
Severe, persistent
-
Symptoms are continual. Nocturnal symptoms are frequent.
-
FEV1 is always abnormal and less than 60% predicted during episodes.
-
Predicted FEV1/FVC reduced by more than 5%
-
Treatment Approach
-
Monitor ABG's and Peak flow or FEV1 closely. If at presentation the PEFR or FEV1 is ≤ 20% of that predicted and does not double after 1 hour of intensive therapy, patient is likely to require intensive treatment, including systemic glucocorticoids and inpatient treatment.
-
Failure of PEFR to improve to ≥ 70% of baseline with emergency treatment suggests need for hospitalization.
Chronic stable asthma
-
Mild, intermittent : Short acting inhaled β2-agonist or glucocorticoid used as needed during exacerbations only
-
Mild, persistent : Low-dose inhaled glucocorticoids ( Budesonide and fluticasone) taken daily in lowest dose necessary to control symptoms. Short acting β2-agonists used as needed for exacerbations
-
Moderate, persistent : Low- to medium-dose inhaled glucocorticoids often combined with long-acting inhaled β2-agonists (Eg: Symbicort, Advair) are used daily. Alternatives include Singulair, mast cell stabilizers and theophylline. Short acting β2-agonsts are used for exacerbations.
-
Severe, persistent: Moderate to high-dose inhaled glucocorticoids and long-acting inhaled β2-agonists are used daily and if needed, oral glucocorticoids are added. Alternatives include Singulair, mast cell stabilizers and theophylline. Anti IgE like Omalizumab is used for highly selected patients who are not controlled on maximal doses of inhaler therapy and have a circulating IgE within a specified range
Specific Treatments in asthma exacerbations:
-
β2-agonists are the primary therapy for acute episodes. Each dose may last 4-6 hrs and major side effect is tremor and tachycardia. Give 2.5 to 5 mg of albuterol by nebulization every 20 minutes for three doses, then 2.5 to 10 mg every one to four hours as needed, or give 4 to 8 puffs by MDI with spacer every 20 minutes for three doses, then every one to four hours as needed. 5 mg dose of albuterol better than 2.5 (Am J Med. 1998 Jul;105(1):12-7)
-
In very severe asthma, only 10% of the inhaled albuterol reaches the bronchi and may need to consider iv salbutamol or sq terbutaline.
-
Treatment with albuterol administered by jet nebulizer, metered-dose inhaler (MDI) with spacer, or dry powder inhaler (DPI) all provide equal resolution in acute situations when doses are matched (Cochrane Database Syst Rev. 2013 Sep 13;(9) ) , (Chest. 2002 Apr;121(4):1036-41) , (Chest. 1993 Mar;103(3):665-72). Also, hourly nebs are as effective as continuous nebulization. It's not the method that is important but the dose and amount of drug that reaches the smaller airways.
-
Anticholinergics: Atrovent nebs can be added for severe bronchodilation, especially in ER. Give 500 mcg by nebulization every 20 minutes for 3 doses, or 4 to 8 puffs by MDI with spacer every 20 minutes as needed for up to 3 hours and then as needed. However, it has very slow onset of action( 60-90mins). Long acting anticholinergics like spiriva is now FDA approved for maintainence therapy in asthma. Ann Am Thorac Soc. 2016 Feb;13(2):173-9.
The utility of anticholenergics in asthma is debatable. Some studies showed an improvement in airflow obstruction when ipratropium is used as an adjunctive treatment to beta2 -agonists for the treatment of acute asthma exacerbation (Thorax. 2005 Sep;60(9):740-6) , (Ann Emerg Med. 1999 Jul;34(1):8-18) , (Am J Respir Crit Care Med. 2000 Jun;161(6):1862-8). However, some studies showed atrovent do not add any therapeutic benefit to the effects of albuterol, nor do they facilitate recovery in patients whose immediate response to sympathomimetics is impaired. (Am J Med. 1997 Jan;102(1):7-13) , (Chest. 1996 Sep;110(3):611-6). Current guidelines strongly recommend not using atrovent beyong the ED during the hospital stay. Asthma Clinical Practice Guidelines , GOLD Guidelines.
-
Systemic glucocorticoids: Prednisone (1 mg/kg PO once daily, usually 40-60mg) or Solumedrol (40-60 mg IV every 6 hours). Dose has to be individualized. In mild to moderate exacerbations, there was no difference between oral and IV steroids. In severe exacerbations, 10-14 days of therapy is adequate.
-
Magnesium sulfate is a smooth muscle–cell relaxant; may also reduce inflammatory bronchoconstriction through actions on mast cells.
- Theophylline may speed resolution after the first hour in 5–10% of patients. Dose required to achieve desired level varies widely from patient to patient owing to differences in drug metabolism. Therapeutic range of serum levels are 10–20 μg/mL at steady state. Due to toxicity issues, this drug is falling out of favour. At plasma levels > 30 μg/mL, there is a risk of seizures and cardiac arrhythmias.
-
Miscellaneous agents:
-
Opiates, sedatives, and tranquilizers should be absolutely avoided in the acutely ill patient with asthma, if they are not intubated
-
Epinephrine ( beta 2 action) drip has been used occasionally, initially nebulised and then iv@2-20mcg/min.
-
In severe bronchospasm, aerosolized bronchodilators maynot reach the smaller airways and hence, SQ terbutaline 0.25 mg every 20 minutes times 3 doses is an option, if patient shows no improvement with duonebs.
-
Ketamine infusion 0.5 to 2 mg/kg per hour, if mechanically ventilated, may help with bronchodilation
-
ECMO as final resort for refractory acidosis
-
Heliox to decrease resistance
-
Paralytics
-
THAM in severe acidosis ( Avoid bicarb drip in severe respiratory acidosis. Bicarb drip will metabolize into CO2+H2O and will lead to worsening PCO2.).
-
Inhaled anesthetic agents like isoflurane are potent bronchodilators
-
Intubation issues in Asthma:
-
Ketamine can make people apneic and may increase secretions. Use caution when using it. However, it also has some bronchodilation properties.
-
Propofol has mild bronchodilator action but can cause hypotension.
-
Maintain an I: E ratio of 1:4 to prevent dynamic hyperinflation.
-
Allow for permissive hypercapnea if airway pressures are too high.
Ventilator Strategies in Asthma:
The major physiologic changes associated with a severe exacerbation are airflow limitation, bronchial hyperresponsiveness, airway closure, loss of elastic recoil, and hyperinflation (or air trapping). These all result from airway narrowing, largely due to bronchoconstriction, although edema and mucus production in the airways also probably contribute to the reduction in airway caliber. In fact, autopsy studies of patients who died of asthma indicate a high incidence of airway obstruction from mucus impaction, which suggests that edema and mucus production may play a more prominent role in severe exacerbations.
The airway narrowing increases the resistance to airflow and requires the patient to work harder to breathe. The increased resistance lengthens the exhalation time required to empty the lung, which leads to air trapping (hyperinflation). In addition, factors such as low pulmonary elastance and persistent activation of the inspiratory muscles contribute to the tendency for air trapping. Hyperinflation stimulates the feeling of dyspnea, impairs gas exchange by increasing dead space, increases the work of breathing, and in extreme cases leads to hemodynamic compromise and barotrauma. Unfortunately, mechanical ventilation, when improperly managed, can exacerbate hyperinflation by increasing the minute ventilation.
Recent research suggests that the pattern of airway narrowing is heterogeneous, leading to lung areas with relatively preserved ventilation near areas with high-grade obstruction. Indeed, some airways may be completely obstructed by severe constriction and mucus impaction and thus may trap gas in the lung at high pressure. The implication is that routine measurements of end-inspiratory and end-expiratory pressure, used to judge the safety of ventilation, may underestimate the amount of trapped gas in the patient, as has been described clinically. Thus, this heterogeneity contributes to the complexity of ventilation and makes it more likely to have unrecognized hyperinflated regions that lead to poor ventilation-perfusion matching, hemodynamic compromise, and increased susceptibility to barotrauma.
Setting the Ventilator Mode
There are clear advantages and disadvantages to both pressure-targeted and volume-targeted strategies.
-
In a pressure-targeted mode the peak inspiratory pressure is limited and the lungs will not be inflated to a pressure above the set peak pressure. This has the advantage of always limiting the amount of hyperinflation. For example, if pressure control is used with an inspiratory pressure of 30 cm H2O, the pressure in the lung will not exceed 30 cm H2O, even if there are occluded airways with trapped gas. Another advantage of a pressure-targeted mode is that if the airway resistance suddenly increases, the patient will not hyperinflate; however, the VT will drop. The problem with a pressure-targeted mode is that if the airway resistance is very high, it will be difficult to deliver an effective VT.
It can be very difficult to provide adequate ventilation (arterial pH < 7.20) to severely obstructed patients with pressure control. This is largely due to the mechanics of delivering a VT against a high resistance with a low pressure limit. The smaller VT also makes it more difficult to deliver aerosolized bronchodilator. Thus, a pressure-targeted mode will provide the safest form of ventilation, but at the expense of decreased CO2 clearance, a lower pH, and less effective aerosol delivery.
It should also be noted that as the patient’s airflow obstruction improves, a high pressure setting with a pressure- targeted mode could lead to a large VT. Thus, as the patient improves, the pressure setting should be reduced accordingly.
-
A volume-targeted approach will better provide a minimal VT by delivering very high flow and pressure that overcome the high airway resistance. This provides better ventilation and aerosol delivery but increases the risk of hyperinflation. Often we monitor plateau pressure after a volume-targeted breath as a surrogate for end-inspiratory lung volume. However, the plateau pressure is an average pressure and will reflect only the pressure in open lung units. Lung areas that have high pressure in the initial part of the inspiratory cycle, and lung segments that become occluded at the end of inspiration, may still be at risk of barotrauma despite a “safe” plateau pressure. Thus, a volume- targeted ventilation mode will better ensure adequate ventilation in severe cases or with abrupt increases in airways resistance, but probably increases the risk of hyperinflation in the asthmatic patient.
-
PRVC: we can set higher pressure limits where we can ensure adequate tidal volumes. At the same time, if resistance improves, the driving pressure will decrease and hence, the tidal volumes will not increase.
Minute Ventilation, Tidal Volume, and Respiratory Rate
The risk of hyperinflation will track directly with the minute ventilation. Most experts recommend limiting VT in ventilated asthmatic patients to 6–10 mL/kg.
Inspiratory Time and Inspiratory Flow
The most critical determinant of hyperinflation in a mechanically ventilated asthmatic patient is the expiration time. The longer a patient exhales, the less gas will be trapped in the lung at end-expiration, which reduces the risk of hyperinflation during inspiration. One can maximize expiratory time for a given minute ventilation by shortening the inspiratory time. In volume-targeted modes this is accomplished by increasing the inspiratory flow rate and using a constant-flow pattern rather than a ramp pattern.
In pressure-targeted modes the flow rate is determined in part by the patient’s inspiratory drive, so there is much less ability to control the flow rate in a pressure-targeted mode. It is important to note that recent research suggests that there is a plateau in expiratory flow after a certain point, so increasing the expiratory time above a certain value has limited benefit. In general, after about 4 seconds of expiration there is nominal gain in reducing hyperinflation.
One must also consider the consequences of a high constant-flow rate during inspiration. Higher airway pressure and a more heterogeneous distribution of ventilation will result when inspiratory flow is high, which can increase the risk of focal areas of hyperinflation and make ventilation less effective by increasing dead space.
Positive End-Expiratory Pressure(PEEP)
The application of PEEP in status asthmaticus is controversial. In patients with emphysema, PEEP can counterbalance the intrinsic PEEP (auto-PEEP) without affecting expiratory flow because of dynamic collapse of the airways and a “waterfall effect.” This can be helpful in patients who are spontaneously breathing, because it improves ventilator triggering. However, in asthmatics the site of increased resistance is in central (less collapsible) airways. Furthermore, asthmatic airways are likely to be stiff (from inflammation) and more resistant to dynamic collapse, and thus will not have the same waterfall effect as in patients with emphysema. If there is no dynamic collapse, then, in theory, the use of PEEP will increase the back-pressure to expiratory flow and result in more hyperinflation.
Most review articles have not recommended the routine use of added extra PEEP in asthmatic patients. However, a recent study suggested that the physiology may be more variable, so some patients respond to PEEP with increased air trapping, some with no change in lung volume, and some with a paradoxical decrease in lung volume. This would suggest that in some patients PEEP can be carefully applied.
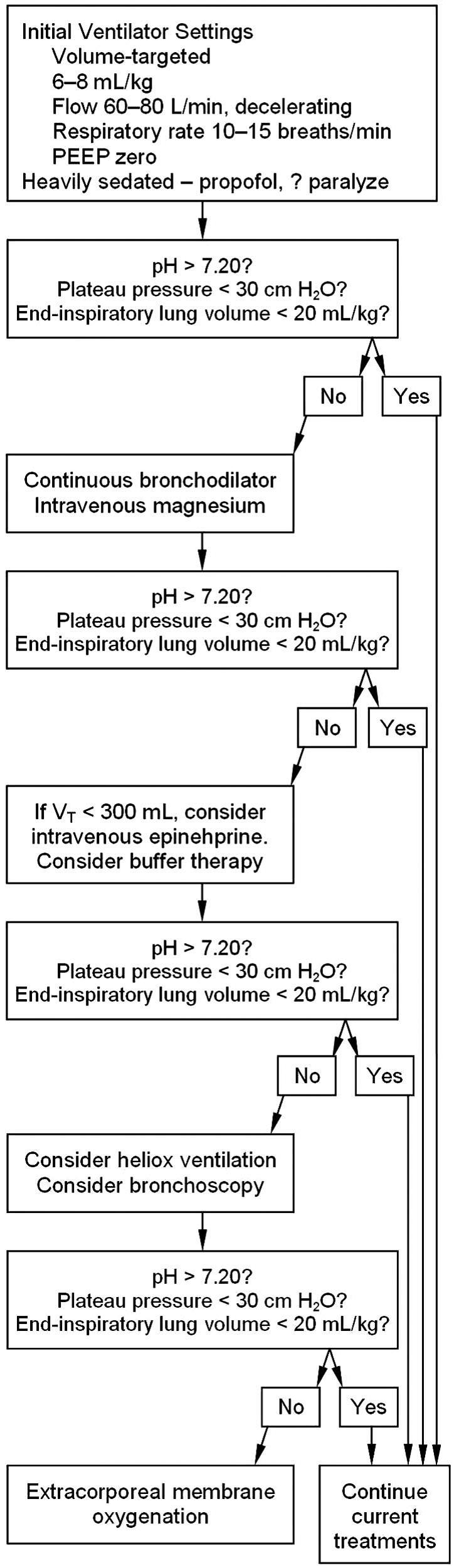
PEARLS:
-
In asthma, uncontrolled, long-standing inflammation may lead to permanent structural changes known as airway remodeling.
-
Spirometry should be performed in patients with suspected asthma to confirm the presence of airway obstruction and reversibility.
-
The methacholine challenge test has a high negative predictive value for asthma, and a negative test can therefore be used to exclude asthma.
-
In patients with difficult-to-control asthma, lifestyle modifications and a trial of acid suppression with a proton pump inhibitor for potential gastroesophageal reflux disease should be considered.
-
Allergic bronchopulmonary aspergillosis: Typically have eosinophilia, elevated serum levels of circulating IgE (total and specific IgE against Aspergillus fumigatus), intermittent pulmonary infiltrates and a positive skin test for Aspergillus. Aspergillus fumigatus can grow in the bronchial secretions of patients with asthma or cystic fibrosis. The organism does not invade tissue, but it results in immune-mediated allergic inflammation. The treatment of ABPA is similar to that of asthma in general, but the initial therapy involves systemic corticosteroids until the disease is controlled; the corticosteroid dosage is then tapered over 6 months, and IgE levels are monitored. Untreated, ABPA can result in permanent lung damage and fibrosis. Itraconazole 200 mg po bid for 4 to 6 mo with a 6-mo taper is recommended as a substitute for prednisone and as a corticosteroid-sparing drug. Itraconazole therapy requires checking drug levels and monitoring liver enzymes and triglyceride and potassium levels.
-
Because of significant drug-drug interaction, toxic serum concentrations of theophylline can be reached in a patient who previously had stable levels but who begins taking an interfering medication, such as a fluoroquinolone antibiotic.
-
Inhaled corticosteroids should be used for long-term control of asthma during pregnancy; all currently available inhaled corticosteroids are considered to be safe during pregnancy.
-
Wheezing may occur in ABPA, eosinophilic pneumonia, and Churg-Strauss syndrome. Note that in uncomplicated asthma, chest radiograph is normal. Findings of infiltrates, striking peripheral blood eosinophilia, and constitutional symptoms, such as fever and weight loss, suggest chronic eosinophilic pneumonia. Asthma with eosinophilia, markedly high serum IgE levels, and intermittent pulmonary infiltrates is characteristic of ABPA. Upper airway and sinus disease, difficult-to-treat asthma, and multisystem organ dysfunction suggest Churg-Strauss syndrome.
-
When peak flow meters are used for managing asthma in outpatient settings, the highest value of PEFR obtained during a 2-week period of stability is regarded as a patient’s personal best. Subsequent values within 20% of the personal best suggests that the disease is stable. PEFR reduction of 20% to 50% from personal best indicates a moderate increase in airway obstruction, whereas a greater than 50% reduction suggests severe airway obstruction.
-
Reactive Airways Dysfunction Syndrome: Exposure to high levels of irritants (for example, chlorine gas, bleach, or ammonia) can result in significant airway injury, which can lead to persistent airway inflammation and dysfunction with airway hyperresponsiveness and obstruction. RADS may subside with time provided that they have no subsequent exposure to the offending agent.
-
Cough-variant asthma: cough can be the predominant or, at times, the only symptom. Patients with cough-variant asthma typically have airway hyperresponsiveness on methacholine challenge testing and can show evidence of obstruction on spirometry, with improvement after inhaled bronchodilators.
-
Adding long-acting β2-agonist inhalers in patients whose asthma is not controlled on low- to medium-dose inhaled corticosteroids is more effective than doubling the dose of inhaled corticosteroids.
-
Exercise-induced Asthma: The symptoms typically occur during or shortly after exercise, peak within 5 to 10 minutes after stopping the activity, and resolve in less than 30 minutes. Short-acting β2-agonists given 10 to 15 minutes before exercise can prevent EIB for up to 3 hours. Long acting β2-agonists are treatment of choice.
-
Vocal cord dysfunction simulates asthma and manifests as recurrent wheezing and stridor. The diagnosis can be confirmed by flow volume loops when the patient is symptomatic, which show an inspiratory cut-off, or laryngoscopy.
-
FEV1 and PEFR can be found from the charts, matched for age and height.
-
Normal PEFR in a 35 year old male is 500ml and female is 400ml
-
BiPAP may help overcome intrinsic PEEP and may reduce work of breathing.
-
PEEP should be set at 60-80% of Auto-PEEP to augment distal airway emptying through splinting airways open
-
Use the largest ET tube in reactive airway disease.
-
Tachycardia will diminish c improvement of obstruction even with beta agonists
-
In adults, no difference between normal and Levalbuterol in reducing tachycardia.
-
Status asthmaticus: Epi 0.3-0.5 mg SQ q 10 min. Can also give inhaled epinephrine.
-
SQ Terbutaline is longer lasting than Epi but slower onset of action.
-
Asthma in prenancy: Magnesium sulphate, albuterol , atrovent and steroids are all safe to use for acute exacerbations.
-